Napcen Bot
We are OnlineNapcen Bot
👋 Greetings! I am Napcen Bot, at your service. How may I be of
assistance to you today?
Build with IndependentWebCrafter


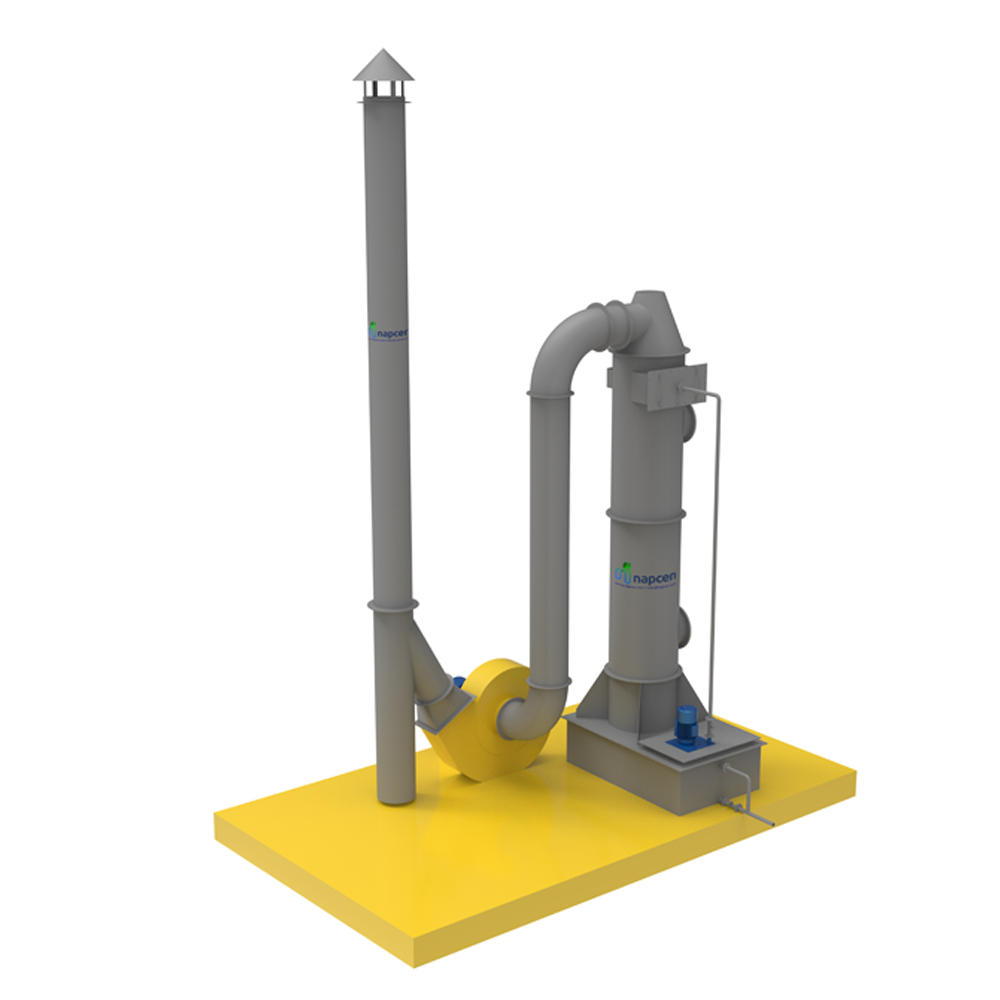

Here's how a nitric acid scrubber typically works:
1.Gas Inlet: The gas stream containing nitric acid enters the scrubber system. This gas stream might be exhaust from processes like metal pickling, chemical manufacturing, or laboratory work.
2.Scrubbing Solution: Inside the scrubber, there is a solution, often referred to as a "scrubbing solution" or "absorbent." This solution is typically a base, such as sodium hydroxide (Na OH) or calcium hydroxide (Ca (OH)2), which can neutralize nitric acid.
3.Contact Zone: The gas stream is passed through the scrubbing solution. In this contact zone, the nitric acid reacts with the base in the solution to form a less harmful compound, typically a nitrate salt.
For example Nitric acid (HNO3) reacts with sodium hydroxide (NaOH) to form sodium nitrate (NaNO3):
HNO3 + Na OH -> NaNO3 + H2O
4.Neutralization: The nitric acid is neutralized as it reacts with the base, effectively removing it from the gas stream.
5.Exit and Disposal: The treated gas stream exits the scrubber, and the resulting nitrate salt is usually dissolved in the scrubbing solution. This solution can then be processed further or disposed of safely, depending on regulatory requirements.
Nitric acid scrubbers are crucial in industries where nitric acid is used or produced because they help control emissions and protect the environment and workers from the harmful effects of nitric acid exposure. Additionally, they help in complying with environmental regulations related to air pollution control.
The design and efficiency of a nitric acid scrubber can vary depending on the specific application, the concentration of nitric acid in the gas stream, and the required emission limits. Scrubber systems may also include additional components like mist eliminators, pumps, and monitoring systems to ensure effective and safe operation.