Napcen Bot
We are OnlineNapcen Bot
👋 Greetings! I am Napcen Bot, at your service. How may I be of
assistance to you today?
Build with IndependentWebCrafter


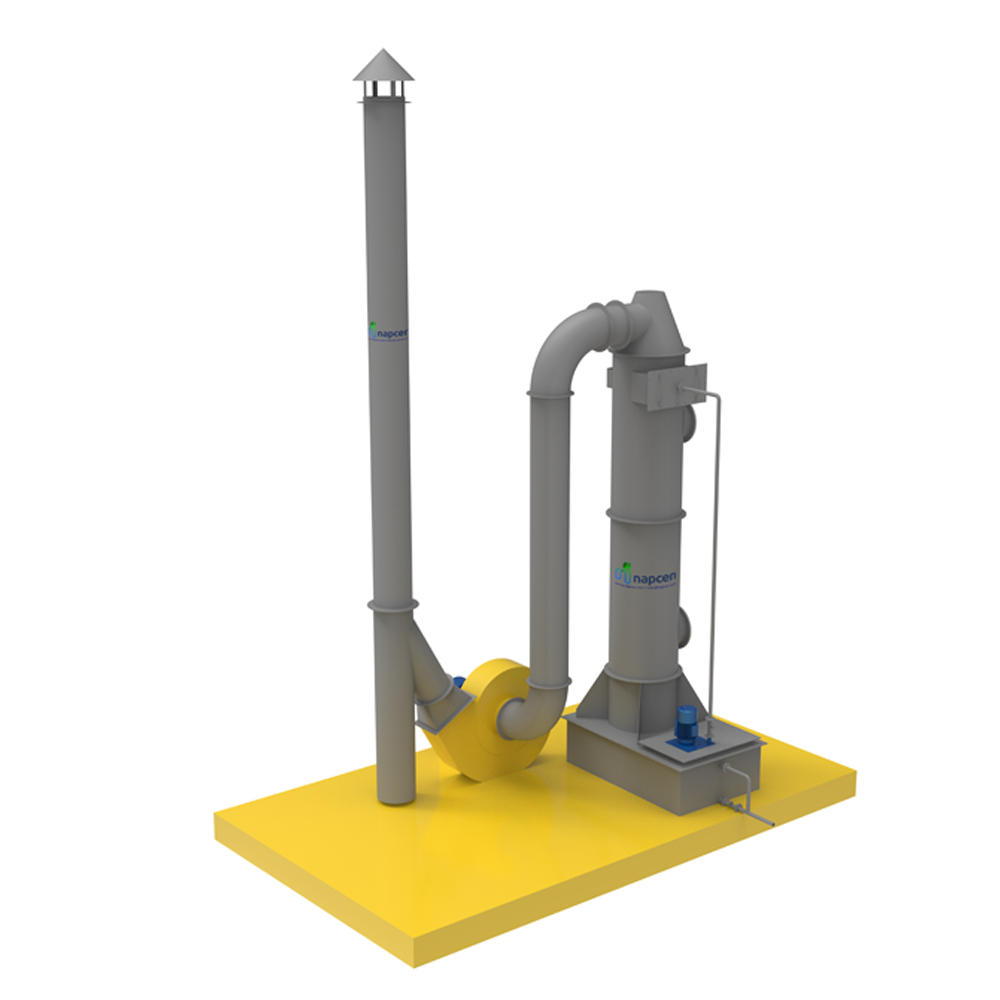

Here's how a typical boiler flue gas scrubber works:
1.Gas Absorption: Flue gas containing pollutants, such as SO2, is directed through a contactor or absorber vessel.
2.Absorbent Solution: In the absorber, the flue gas comes into contact with an absorbent solution, usually a liquid slurry or a chemical reagent. The most common absorbent is limestone (calcium carbonate), which reacts with the SO2 to form calcium sulfite or sulfate.
3.Chemical Reaction: The chemical reaction between the absorbent and SO2 converts the sulfur dioxide into a less harmful compound. This reaction is typically represented as follows:
SO2 + CaCO3 (limestone) -> CaSO3 (calcium sulfite) + CO2
4.Removal of Solid Byproducts: The calcium sulfite or sulfate formed in the reaction is separated from the absorbent solution in a solid-liquid separation unit. The remaining liquid can be recirculated for further use.
5.Waste Disposal: The solid byproducts are typically treated as waste and disposed of or used in other applications
The result of this process is cleaner flue gas with reduced sulfur dioxide emissions. Boiler flue gas scrubbers are essential in many industries to meet air quality standards and minimize the environmental impact of combustion processes. They are particularly important for coal-fired power plants and other facilities that burn high-sulfur fuels.
Different types of flue gas scrubbers exist, including wet scrubbers, dry scrubbers, and semi-dry scrubbers, each with its own advantages and disadvantages.
The choice of scrubber technology depends on factors such as the specific pollutants to be removed, the type of fuel being burned, and regulatory requirements.
Overall, boiler flue gas scrubbers play a crucial role in reducing air pollution and protecting the environment by capturing harmful emissions from industrial processes.